Finding Humanity in Science
What drives someone to throw aside selfish pursuits to focus on projects with true philanthropic impact? Here, the winners of the 2015 Humanity in Science Award provide their answers.

The 2015 Humanity in Science Award (www.humanityinscienceaward.com) was presented jointly to Peter Seeberger and Andreas Seidel-Morgenstern, directors at two collaborating Max Planck institutes in Germany, by our sister publication The Analytical Scientist. Their groundbreaking work in drug synthesis also won both scientists a spot on our 2015 Power List. By coupling flow chemistry with advanced chromatography methods, Seeberger and Seidel-Morgenstern were able to manufacture artemisinin-based therapies – the most effective drugs to treat malaria – from plant waste material, air and light. The science is innovative and exciting, and the potential impact of their project – and the concepts born from it – could really shake things up in the pharmaceutical industry.
The key active pharmaceutical ingredients (APIs) of all artemisinin combination therapies are produced in one or two chemical steps from artemisinin (see Figure 1). The majority of artemisinin (~200 tons per year) is extracted from the Sweet Wormwood plant (Artemisia annua) cultivated for the purpose, and prices fluctuate with harvest yields, driving up prices. With demand outstripping supply, up to 50 percent of anti-malaria drugs sold in Africa and Asia are counterfeit – useless and sometimes toxic.
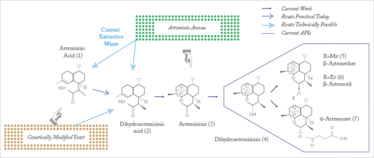
Seeberger came up with a process for photochemical continuous synthesis of artemisinin from a waste product of the plant, DHAA. Yields were low at first – 40 percent yield at 200g per day – but careful optimization resulted in a greatly simplified process and a significantly improved yield. To demonstrate the power of the fully continuous synthesis/purification regime, Seeberger and Seidel-Morgenstern developed a continuous three-stage, multi-column chromatographic/crystallographic purification method for artemisinin-derivative α-artesunate (see Figure 2).
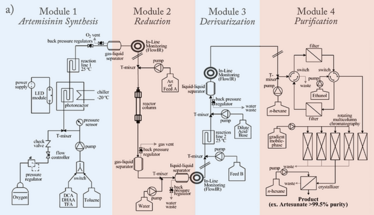
The combined process produces artemisinin of greater than 99.9 percent purity and is now being implemented in a pilot plant in Vietnam. Not only does the new process enable production of less expensive anti-malaria medications, it also increases participation of developing nations in the value chain of drug production.
Now, let’s focus on the personal stories of the duo that led the project, to discover what seeds humanity in science.
You can read the full submission to the Humanity in Science Award online: www.humanityinscienceaward.com
Using Entrepreneurship and Chemistry for Good
Peter Seeberger, Professor and Director of the Max Planck Institute of Colloids and Interfaces, Potsdam, Germany.

How did you get into chemistry?
I grew up in Nuremberg in Bavaria and was the first member of my family to go to university. I guess I was a good high school student, because I qualified for the highest possible scholarship for Bavaria, something that is awarded to just a select few. I could have studied anything, but I chose chemistry.
I then had to do my mandatory national service in the German army, which further motivated my pursuit of chemistry – the armed forces were definitely not for me. I studied both chemistry and business to begin with, but I eventually focused on chemistry because it gave me the chance to stand out from the crowd. I studied chemistry for three years at University Erlangen-Nuremberg with a full scholarship. The program normally took five to six years to complete, but after three years I had finished and was nominated for a full graduate scholarship to go to the US for a year. I applied to both Berkeley and Colorado universities and ended up going to Colorado, which was great as I like skiing...
I finished my PhD in Colorado working with Marvin Caruthers – a member of the US National Academy who famously automated DNA synthesis and set up many companies, including Amgen. Working with him made me realize that doing very good chemistry could also help you to do very good biology. The idea of starting up companies was also interesting.
So you moved again?
Right. Bruce Merrifield won a Nobel Prize in Chemistry in 1984 for chemical peptide synthesis on a solid matrix and I thought I could do something similar for carbohydrates. To prepare myself, I applied to work with the best-known carbohydrate chemist of the time – Sam Danishefsky, professor at Memorial Sloan Kettering Cancer Center and Columbia University. He accepted me into his lab in New York, where I worked extremely hard – 18-hour days, seven days a week – for two years. I focused on developing methods for carbohydrate synthesis.
I’d already lined up a job in Germany as an assistant professor, but before I could accept it Danishefsky called me to his office at 1am on December 23 and asked me what I’d be doing after leaving his lab. He encouraged me to apply to Massachusetts Institute of Technology (MIT) – and I did. They invited me to give a talk and then I had a day-long interview. The following day I received an offer to be an assistant professor at MIT. I accepted.
Why was Danishefsky so keen to push you to MIT?
Danishefsky encouraged many of his people to apply to leading institutions. I’d published 12 papers with him and he seemed to think I would be a good match for MIT.
Moving to MIT was the best career decision I ever made, so I’m thankful to Danishefsky for pointing me in the right direction. Often, your choices in life are due to the influence of your mentors and role models. Without their influence, I would never have considered applying to top universities. When you come from Bavaria, places like Harvard and MIT are pretty far away – and not just in distance.
I did not know what to expect at MIT, but it worked out OK. I remember that in the first three days of starting my job, one of my colleagues asked me to go to the faculty lunch room and the provost said to me, “Young man, what do you think of your chances of getting tenure?” I replied that I had no idea, but I guess I was lucky because after about four years I was promoted to tenured professor at MIT at the age of 35.
Sometime later, ETH (the Swiss Federal Institute of Technology) in Zurich made me an offer I could not refuse. I’d been in the USA for 13 years and thought I would probably stay there my whole life. If I turned down ETH, I thought it would be difficult to return to Europe.
Initially, I didn’t find life at ETH easy because I’d been Americanized both in the way I spoke English and in my etiquette. It was a learning process for both sides I think. I was there for six years, met my partner (who was a professor in Berlin) and had a daughter. The commute between Zurich and Berlin needed fixing.
The Max Planck society offered me a job to take over a directorship at the Institute for Medicine in Heidelberg. It was a good offer, but it would not improve my family situation (travelling between Heidelberg and Berlin is actually worse than travelling from Zurich to Berlin). However, the people at Max Planck were persistent and suggested that I join an institute in Potsdam where they would erect a new building for us. I have to say I was anxious about the move. When I left MIT it was one of the most difficult days of my life because I was not sure whether I made the right decision. I was in a similar situation and knew there would be a lot of things I would miss about Switzerland. That said, I have been lucky in life and felt it would turn out well.
What brought you such success?
First of all, you have to pick a good area to work in. Glycosciences is a fantastic area with seven Nobel Prize winners up until the early 1970s – and glycans are everywhere. The advances in molecular biology of the mid-1970s and the new-found ability to manipulate DNA for proteins meant carbohydrates took a back seat and the technologies for enabling glycomics and glycobiology were lacking. I had expertise in DNA and peptide and carbohydrate chemistry that no one else had at the time. Many said that my idea for automated synthesis of carbohydrates wouldn’t work, but it was a smart choice given my background.
I also work really hard – I’m very driven. I don’t think I’m more intelligent than the next guy, but perhaps I am able to see interesting areas that enable long-term programs rather than just solving little puzzles.
And that approach fits in with your work at Max Planck where you are building platforms?
Yes – and that’s how I got interested in flow chemistry, which is part of the work we received the Humanity in Science Award for. It’s something I’ve been involved in since my days at MIT, where I remember hearing a talk by a physicist who had begun working on flow chemistry while he was in Germany. He talked about how you do chemistry in pipes instead of buckets, and that really appealed to me.
I started building systems and platforms. And we slowly began to get involved in medicine – after all, though my students are very well trained in carbohydrate chemistry, they need experience with drug molecules to improve their employment prospects! In fact, most of the people we train go into industry; more than 200 of them have left to get really good jobs. But I’ve also seen 47 professors come out of my lab.
It’s fantastic to have the opportunity to convince talented young chemists to work with me. I give directions to make sure we get to a certain point, but the important work is done and implemented by the young scientists we train. If our students weren’t as diligent, things could have gone in a very different direction.
What drove you to explore antimalarial drug manufacture?
Early on in your career, you want to make sure you publish the best possible papers. Then one day you ask yourself: “Do I want to publish another paper or do I want to make a real impact?” I knew that if I wanted to have impact on a global scale, I needed to work on something that could improve other people’s lives, particularly those who have little chance themselves. So, that’s why I started to think about things like malaria and HIV (we’re also working on cheap antibiotics and anticancer medications). The artemisinin project was the first step in that direction. I’d usually work on vaccines – a cheap means to prevent disease – but with artemisinin, it became clear that the medication is there, but a huge part of the global population (the ones who most need it) can’t afford to buy it. You can talk to people who travel to Africa and they will tell you that they had to lock up their antimalarial drugs to prevent the cleaning staff from stealing them. The inequality at the heart of the problem is just wrong.
I thought, “we can do something about this problem”. But it wasn’t enough to simply publish a new method – we had to take it further than that. It is very difficult to translate published research into something that can be implemented and taken to market; it’s very frustrating to see good existing solutions that are simply not being implemented in developing countries. And it’s sad that so many people are leaving Africa because of the bad conditions – the real solution is to help these people have better lives without them having to leave. I can only make a small contribution, but one of our goals is to convince governments to do something regarding technology, but even there I see some reluctance.
But much of the world is governed by economics – and that has to change, right?
When the artemisinin story first broke, it was all over the news. People said, “This is great, congratulations!” But without a lot of effort, it won’t see the light of day because the big companies will try to bury it. Pharma companies are traded on the stock market so they have to make profits. One of the big questions of our times is how are we going to evolve drugs in the future? For example, I know that if any of us were investing in a pension fund, we’d expect the fund managers to make the best decision about putting our money into a drug company or into a car company. Is the drug company expected to give a big discount or free drugs to Africa? What about the car company? Does it have no obligation to give a discount? Right now, the system is not really geared towards improving health for a large part of the global population.
Unfortunately, I don’t have a total solution to that problem – just a small piece of the puzzle. But wouldn’t it be great if some really smart people stopped to think about changing the world of pharmaceuticals – not just how they are manufactured, but also how they evolve and how they are paid for. I do believe in a free market, but the population of poor people around the world is growing and being left behind – that can’t be right. I don’t think it’s good to live in a world where very few people are very rich and a greater number of people have nothing. If I look back on my own life in Germany in the 1970s and 1980s, there were very few poor people and very few rich people. We see in many countries that the middle classes are being dissolved – the polarization of society. I don’t think it would be fun to be part of these societies – even if you are rich.
Preparing to Change the World
Andreas Seidel-Morgenstern, Director of the Department of Physical and Chemical Foundation of Process Engineering, Max Planck Institute for Dynamics of Complex Technical Systems, Magdeburg, Germany.

Take us back to the early days...
I grew up and worked in what was East Germany – right up until I got my PhD, which I did in 1987 in the former Academy of Sciences’ Institute of Physical Chemistry in East Berlin. It was an interesting time, but also very political. In fact, my scientific career was more or less over after I completed my PhD because I refused to become a member of the East German communist party. Later in life, I read my Stasi [The Ministry for State Security] file, which noted that I wasn’t loyal and should not benefit from promotion. At the time, I did not really appreciate how strict the system was.
Then in 1989, the wall came down. It was a new world for me. I had my PhD and I wondered what I should do next. Because the “iron curtain” no longer trapped me, I contacted the Technical University in West Berlin where a colleague (who later became my boss) helped me become a more active member of the German chemical engineering community. Later, I thought I should move to an English-speaking country to see more of the world.
So you moved to the USA?
Right. My wife is a chemical engineer as well – we met in the old East Germany and we already had a family, but we were not married. One day she came home and said the company she was working for was collapsing, but there was an opportunity in Tennessee, USA. That evening I was thinking about a few papers I’d read from a guy in Tennessee – I could not recall his name so I searched my files and found him: Georges Guiochon, University of Tennessee, Knoxville.
The next day I wrote him a letter to ask if he had any positions open. About three weeks later the answer came back: “yes.” I included a research project idea in the letter – a smart move – Georges liked it. He offered me a post-doc position and my wife and I married to make it easier to move to the USA with the children as a family.
Why do you think Georges was so keen to work with you?
Georges’ mathematical oriented views on chemistry and in particular chromatography meant he always had very good foreign experts. I guess he liked to work with anyone – foreigners included – that fit in with his strategy. I brought a new idea that was related to my PhD (how to calculate competitive adsorption isotherms) and I think he saw this field as a chance to enrich his own scope.
I could have stayed longer in the USA, but I applied for and received a grant from the German Science Foundation to support three-years at the Technical University of Berlin to do my habilitation. At the same time, Georges suggested that I should stay in the USA – this was a tough decision. However, we returned to Germany. We had young children and were still very poor; it was financially risky to remain in the USA – and my widowed mother was alone back home in Germany.
So, in 1992, we returned to Germany and I did a relatively rapid habilitation. I had many results so it was easy. Unfortunately, I did it too quickly because the grant rule agreement essentially said, “when you finish, we stop paying you”. To make ends meet, I got a job with Schering in Berlin, but I wasn’t there for very long; my boss encouraged me to apply for academic positions, noting an opportunity in Magdeburg, which I took.
And that was the connection to the Max Planck Society?
Yes. Three years later, there was an unexpected situation in Magdeburg. Germany was united and the Max Planck Society (supported by taxes) started to invest in the former East Germany to ensure an even distribution of funding throughout the German states. Up until then, Magdeburg and the federal state Saxony-Anhalt had not received much support. The society decided to form a new institute on Dynamics of Complex Technical Systems, which now houses more than 200 people.
I became director of the institute in 2002, and we now have many projects in various engineering areas – chromatography is just one of them. By training, I am a reaction engineer and so we also do a lot of analyzing and quantifying reaction processes, which broadens our separation science based scope.
The Max Planck Society meets once or twice a year at annual meetings, and that’s how I met Peter Seeberger. I quickly realized we were well matched; his group was strong in chemistry whereas we had expertise in designing continuous separation processes. We connected and now have quite a few stories to tell.
How do you work with Peter?
Peter’s group focuses on certain target molecules and constantly comes up with new and fascinating chemistries. Very often, his reaction pathways are challenging (and very clever), but connecting them directly to our separation processes can be difficult. We started studying a simple model reaction. Then we worked intensively on artemisinin and artesunate. Currently, we are looking together for new target molecules to further contribute to this area.
My overarching goal is to develop universal methods that can be applied generically. Devising strong, widely applicable technologies is what interests us the most. Nevertheless, we are not naïve; we know that every separation problem brings its surprises. We need sufficient flexibility to fine-tune what we do.
The malaria story is wonderful. Our job was to establish a separation concept to isolate a valuable component from a complex mixture. And we have many other examples of similar problems in biotechnology. Typically, a single product will exist alongside many conflicting by-products. How can we structure the separation problem in a more generic way? If you think of chromatography, you could have a sample containing 100 components, but component 17 is your target. How do you get it? We consider this a pseudo ternary separation problem. One to 16 forms a big fraction before the target – the first fraction. The second fraction is your target – component 17 in a very narrow window, followed by another big third fraction (18–100). If you find a process that looks at the problem in the same way – for example, ternary simulated moving bed (SMB) chromatography – you can tune various pump flow rates representing the crucial process parameters to enable you to isolate any target from any mixture. Of course, in practice you have to connect several process steps together and you need to recycle streams if you want to be efficient and not lose valuable materials. That’s characteristic of our way of looking at problems.
What would you like to leave behind for future generations?
We now have expertise in separating enantiomers and I would like to expand that knowledge into new chiral compounds. Agrochemistry, for example, is an interesting area. We apply large amounts of agrochemicals onto fields, but they are often chiral meaning that we could often be wasting 50 percent of these products simply because we don’t understand their exact mechanisms and we do not have access to the active form. If we separate these mixtures into pure enantiomers, we will find that one will be more effective than the mixture. These are very cheap molecules compared with pharmaceuticals, so the agrochemical industry is not keen on using costly SMB technology, complicated columns, or high-pressure pumps. They need cheap separation technology and that leads us away from chromatography and into crystallization. Now, we are working very intensively on a fantastic process called preferential crystallization that might be suitable for resolving the enantiomers of chiral agrichemicals, which should offer a much cheaper solution.
In general, I dream of creating and developing more efficient and cheaper separation alternatives that take greater advantage of molecular interactions between solid and liquid phases.
What do you find most scientifically rewarding?
From a scientific perspective, I like to to quantify processes. Organic synthesis chemists like Peter validate the success of their synthesis reactions using proven analytical methods. That’s not enough for me. Quantification means finding ways to predict the outcomes of processes and to design them with respect to a specific objective; for example, to decide how big a reactor or a chromatographic column should be to do a certain job in an optimal manner. To reach such goals we need to know many details – the rates of the reactions taking place in the reactor, for example. To understand these rates, we need to understand molecular aspects that create them, which requires a broader perspective that bridges multiple scales.
Thermodynamics teach us that our fantastically organized planet is not a stable system – it can’t last forever. The multiscale approach looks at this problem in a quantitative and systematic way, covering multiple time and length scales. It proceeds from molecular models, macroscopic kinetic and thermodynamic models, models for specific apparatuses, calculations on the plant level to simulations evaluating process impacts at the earth level and beyond. The challenge is to develop tools that transfer the knowledge to the next level without losing too much precision and resolution. This multiscale view, which attracts increasing attention within the various communities, needs more research on all levels. People who have expertise in connecting various scales will be important and highly sought after in the future.
In addition, I think that researchers should be happy to work intensively with young people, who always ask new questions. Currently, I teach a lot and I like it very much. I fully support the classical view of Wilhelm von Humboldt that universities always need to balance and unite teaching and research. Indeed, besides doing good research, we should all endeavour to be good teachers.
Peter Seeberger’s research covers a broad range of topics from engineering to immunology. He is a director at the Max-Planck Institute for Colloids and Surfaces in Potsdam and Professor at the Free University of Berlin. Through his work in the area of neglected diseases, Peter has also become involved in philanthropic causes – he is a co-founder of the Tesfa-Ilg ‘Hope for Africa’ Foundation that aims to improve healthcare in Ethiopia.
After receiving his PhD from the Academy of Sciences in Berlin and working as a postdoctoral fellow at the University of Tennessee, Andreas Seidel-Morgenstern defended a Habilitation at the Technical University, Berlin before working for Schering AG. In 2002 he joined the Max Planck Institutes, where he is head of the Physical and Chemical Foundations of Process Engineering group. Andreas is interested in heterogeneous catalysis, the development of new reactor concepts, crystallization, adsorption and preparative chromatography. The results of his work are published in almost 400 research papers.